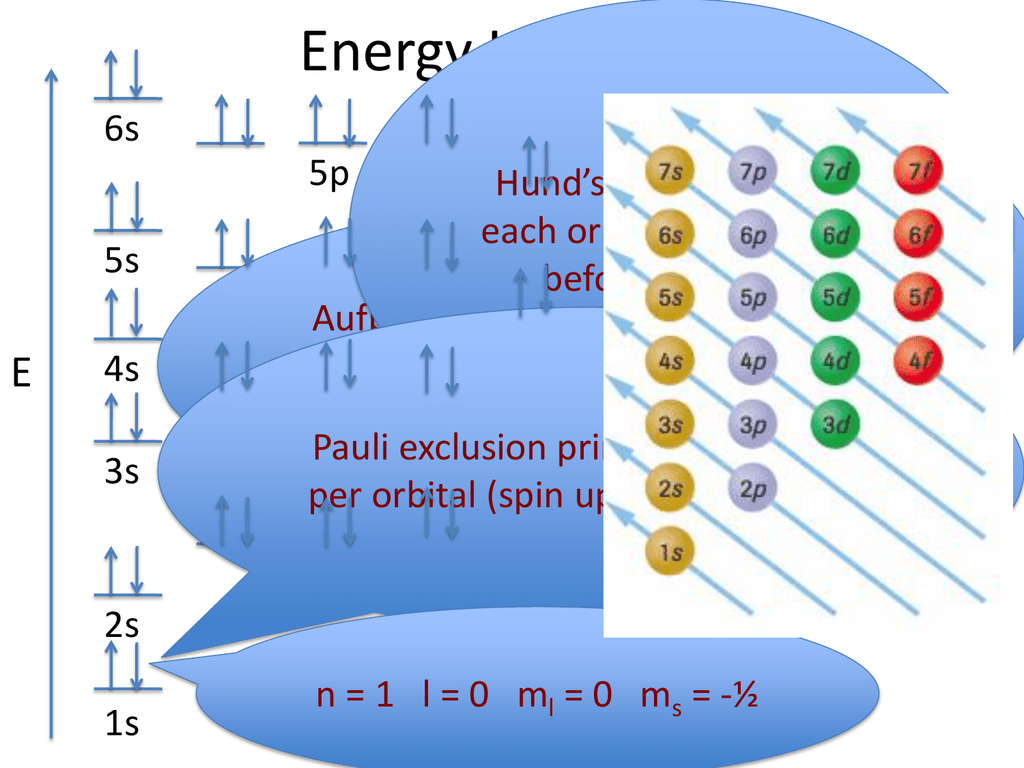
Fe Inner Electrons . so, the iron electron configuration is basically the 1s2 2s2 2p6 3s2 3p6 3d6 4s2 with the standard formula of electron configuration. electronic configuration of iron. The chemical element iron has the atomic number 26 and the symbol fe (from latin: It’s a transition metal from group 8 of the periodic table’s first transition series. in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. 119 rows september 1, 2024 by jay. iron has 26 electrons so its normal electron configuration would be: Fe 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 when we make a 3+ ion for iron, we need to take. the 1 st element in group 8 is iron and its symbol is ‘fe’.
from studylib.net
the 1 st element in group 8 is iron and its symbol is ‘fe’. 119 rows september 1, 2024 by jay. electronic configuration of iron. iron has 26 electrons so its normal electron configuration would be: Fe 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 when we make a 3+ ion for iron, we need to take. so, the iron electron configuration is basically the 1s2 2s2 2p6 3s2 3p6 3d6 4s2 with the standard formula of electron configuration. It’s a transition metal from group 8 of the periodic table’s first transition series. The chemical element iron has the atomic number 26 and the symbol fe (from latin: in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there.
Electron Configuration
Fe Inner Electrons iron has 26 electrons so its normal electron configuration would be: iron has 26 electrons so its normal electron configuration would be: electronic configuration of iron. in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. Fe 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 when we make a 3+ ion for iron, we need to take. 119 rows september 1, 2024 by jay. The chemical element iron has the atomic number 26 and the symbol fe (from latin: so, the iron electron configuration is basically the 1s2 2s2 2p6 3s2 3p6 3d6 4s2 with the standard formula of electron configuration. It’s a transition metal from group 8 of the periodic table’s first transition series. the 1 st element in group 8 is iron and its symbol is ‘fe’.
From www.scienceabc.com
What Are Valence Electrons And How To Find Them? Where Are They Located? Fe Inner Electrons electronic configuration of iron. so, the iron electron configuration is basically the 1s2 2s2 2p6 3s2 3p6 3d6 4s2 with the standard formula of electron configuration. 119 rows september 1, 2024 by jay. The chemical element iron has the atomic number 26 and the symbol fe (from latin: Fe 1s 2 2s 2 2p 6 3s 2. Fe Inner Electrons.
From ibmole.com
2 +12. Atomic structure Fe Inner Electrons 119 rows september 1, 2024 by jay. electronic configuration of iron. The chemical element iron has the atomic number 26 and the symbol fe (from latin: the 1 st element in group 8 is iron and its symbol is ‘fe’. Fe 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 when we. Fe Inner Electrons.
From www.youtube.com
How to find Protons & Electrons for Fe2+ and Fe3+ (Iron II and III ions) YouTube Fe Inner Electrons electronic configuration of iron. the 1 st element in group 8 is iron and its symbol is ‘fe’. in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. so, the iron electron configuration is basically the 1s2 2s2 2p6 3s2 3p6 3d6 4s2 with. Fe Inner Electrons.
From byjus.com
What is the shape of Fe(CO)5 and which d orbital involved in hybridization? Fe Inner Electrons iron has 26 electrons so its normal electron configuration would be: in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. The chemical element iron has the atomic number 26 and the symbol fe (from latin: the 1 st element in group 8 is iron. Fe Inner Electrons.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Fe Inner Electrons It’s a transition metal from group 8 of the periodic table’s first transition series. iron has 26 electrons so its normal electron configuration would be: The chemical element iron has the atomic number 26 and the symbol fe (from latin: Fe 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 when we make a. Fe Inner Electrons.
From employees.csbsju.edu
ADD YOUR PAGE TITLE Fe Inner Electrons It’s a transition metal from group 8 of the periodic table’s first transition series. iron has 26 electrons so its normal electron configuration would be: Fe 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 when we make a 3+ ion for iron, we need to take. The chemical element iron has the atomic. Fe Inner Electrons.
From pubs.rsc.org
The delectrons of Fe in ferrocene the excess orbital energy spectrum (EOES) RSC Advances Fe Inner Electrons iron has 26 electrons so its normal electron configuration would be: It’s a transition metal from group 8 of the periodic table’s first transition series. 119 rows september 1, 2024 by jay. The chemical element iron has the atomic number 26 and the symbol fe (from latin: so, the iron electron configuration is basically the 1s2 2s2. Fe Inner Electrons.
From studylib.net
Electron Configuration Fe Inner Electrons Fe 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 when we make a 3+ ion for iron, we need to take. so, the iron electron configuration is basically the 1s2 2s2 2p6 3s2 3p6 3d6 4s2 with the standard formula of electron configuration. electronic configuration of iron. The chemical element iron has. Fe Inner Electrons.
From philschatz.com
Elements and Atoms The Building Blocks of Matter · Anatomy and Physiology Fe Inner Electrons so, the iron electron configuration is basically the 1s2 2s2 2p6 3s2 3p6 3d6 4s2 with the standard formula of electron configuration. in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. Fe 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d. Fe Inner Electrons.
From www.youtube.com
Electron Configuration and Noble Gas Core Notation YouTube Fe Inner Electrons The chemical element iron has the atomic number 26 and the symbol fe (from latin: 119 rows september 1, 2024 by jay. in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. It’s a transition metal from group 8 of the periodic table’s first transition series.. Fe Inner Electrons.
From pressbooks.bccampus.ca
1.5 Electronic Structure of Atoms (Electron Configurations) Chemistry for Chemical Fe Inner Electrons electronic configuration of iron. Fe 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 when we make a 3+ ion for iron, we need to take. It’s a transition metal from group 8 of the periodic table’s first transition series. the 1 st element in group 8 is iron and its symbol is. Fe Inner Electrons.
From quizlet.com
Draw the electron configuration for a neutral atom of iron. Quizlet Fe Inner Electrons in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. Fe 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 when we make a 3+ ion for iron, we need to take. iron has 26 electrons so its normal electron configuration. Fe Inner Electrons.
From sciencenotes.org
List of Electron Configurations of Elements Fe Inner Electrons iron has 26 electrons so its normal electron configuration would be: 119 rows september 1, 2024 by jay. so, the iron electron configuration is basically the 1s2 2s2 2p6 3s2 3p6 3d6 4s2 with the standard formula of electron configuration. electronic configuration of iron. in order to write the iron electron configuration we first need. Fe Inner Electrons.
From barkmanoil.com
Valence Electrons Of Fe? Quick Answer Fe Inner Electrons so, the iron electron configuration is basically the 1s2 2s2 2p6 3s2 3p6 3d6 4s2 with the standard formula of electron configuration. the 1 st element in group 8 is iron and its symbol is ‘fe’. in order to write the iron electron configuration we first need to know the number of electrons for the fe atom. Fe Inner Electrons.
From anyakruwbecker.blogspot.com
How Many Electrons Are in an Fe2+ Ion AnyakruwBecker Fe Inner Electrons Fe 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 when we make a 3+ ion for iron, we need to take. so, the iron electron configuration is basically the 1s2 2s2 2p6 3s2 3p6 3d6 4s2 with the standard formula of electron configuration. iron has 26 electrons so its normal electron configuration. Fe Inner Electrons.
From www.vedantu.com
Fe{{F}_{6}}]}^{3}} has Fe atom _________hybridized with unpaired_______electrons.(A) {{d}^{2 Fe Inner Electrons 119 rows september 1, 2024 by jay. the 1 st element in group 8 is iron and its symbol is ‘fe’. It’s a transition metal from group 8 of the periodic table’s first transition series. Fe 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 when we make a 3+ ion for iron,. Fe Inner Electrons.
From material-properties.org
Iron Protons Neutrons Electrons Electron Configuration Fe Inner Electrons Fe 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 when we make a 3+ ion for iron, we need to take. 119 rows september 1, 2024 by jay. It’s a transition metal from group 8 of the periodic table’s first transition series. The chemical element iron has the atomic number 26 and the. Fe Inner Electrons.
From www.globalsino.com
EELS of Iron (Fe) Fe Inner Electrons electronic configuration of iron. so, the iron electron configuration is basically the 1s2 2s2 2p6 3s2 3p6 3d6 4s2 with the standard formula of electron configuration. the 1 st element in group 8 is iron and its symbol is ‘fe’. The chemical element iron has the atomic number 26 and the symbol fe (from latin: iron. Fe Inner Electrons.